Multiple Choice
The most energetic electron in any atom at the beginning of a period of the periodic table is in:
A) an 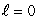 state
state
B) an 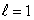 state
state
C) an 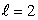 state
state
D) an n = 0 state with unspecified angular momentum
E) an n = 1 state with unspecified angular momentum
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: Electrons in a certain laser make transitions
Q33: Possible values of the principal quantum
Q35: In the relation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3150/.jpg" alt="
Q36: In connection with x-ray emission the
Q38: If electrons did not have intrinsic angular
Q39: An electron in an atom is
Q41: Six electrons are in a two-dimensional square
Q44: In a helium-neon laser, the laser light
Q57: The purpose of the mirrors at the
Q60: The most energetic photon in a continuous