Multiple Choice
How many atoms in this molecule have a trigonal planar geometry? 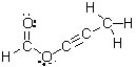
A) one
B) two
C) three
D) four
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which compound has 24 valence electrons?<br>A) C<sub>3</sub>H<sub>6</sub>O<br>B)
Q2: Which statement is CORRECT concerning the cyanide
Q3: What is the formal charge on the
Q5: A ClO<sub>2</sub><sup>-</sup><sup> </sup>ion has _ valence electrons.<br>A)
Q6: How many atoms in this molecule have
Q7: Which molecule does NOT have a lone
Q8: The Lewis structure of the cyanide ion
Q9: Which compound has an expanded octet around
Q10: Based on the Pauling electronegativities,an Fr-F bond
Q11: What is the electronic geometry around the