Multiple Choice
Consider the following images.The reactions occurring in each are shown in the choices. 



A B C D
Which of the following is an example of an electron transfer reaction?
A) Pb(NO3) 2(aq) + Na2SO4(aq) 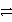 2 NaNO3(aq) + PbSO4(s)
2 NaNO3(aq) + PbSO4(s)
B) H2CO3(aq) 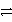 H2O(l) + CO2(g)
H2O(l) + CO2(g)
C) Ca(s) + 2HCl(aq) 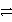 CaCl2(aq) + H2(g)
CaCl2(aq) + H2(g)
D) Zn(s) + Cu2+(aq) 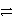 Zn2+(aq) + Cu(s)
Zn2+(aq) + Cu(s)
E) Both c and d are electron transfer reactions.
Correct Answer:

Verified
Correct Answer:
Verified
Q3: What happens in a galvanic cell?<br>A)Cations are
Q15: Oxidation-reduction reactions are also known as...<br>A)electron-transfer reactions<br>B)proton-transfer
Q22: Silver metal will not react with hydrochloric
Q27: Which of the following is the correct
Q27: Match each term with its correct classification:<br>Proton
Q28: Which of the following is the correct
Q28: Reduction can be defined as...<br>A)a increase in
Q30: What is the oxidation number of chromium
Q35: Which of the following cannot be a
Q36: Balance the following redox equation in acidic