Multiple Choice
Which of the following is the correct net ionic equation for the redox reaction between nickel ion and metallic silver and the correct direction that is favored? The half reactions are:
Ag+ + e- 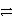
Ag(s) Ni2+ + 2e- 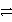
Ni(s) Ag+ is a stronger oxidizing agent than Ni2+.
A) Ni2+(aq) + Ag(s) + e- 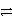 Ni(s) + Ag+ (aq) forward
Ni(s) + Ag+ (aq) forward
B) Ni2+(aq) + Ag(s) + e- 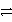 Ni(s) + Ag+(aq) reverse
Ni(s) + Ag+(aq) reverse
C) Ni2+(aq) + Ag(s) 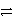 Ni(s) + 2 Ag+(aq) reverse
Ni(s) + 2 Ag+(aq) reverse
D) Ni2+(aq) + 2 Ag(s) 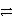 Ni(s) + 2 Ag+(aq) forward
Ni(s) + 2 Ag+(aq) forward
E) Ni2+(aq) + 2 Ag(s) 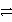 Ni(s) + 2 Ag+(aq) reverse
Ni(s) + 2 Ag+(aq) reverse
Correct Answer:

Verified
Correct Answer:
Verified
Q3: What happens in a galvanic cell?<br>A)Cations are
Q11: What evidence suggests that Na<sup>+</sup> ions are
Q15: Oxidation-reduction reactions are also known as...<br>A)electron-transfer reactions<br>B)proton-transfer
Q22: Silver metal will not react with hydrochloric
Q27: Which of the following is the correct
Q27: Match each term with its correct classification:<br>Proton
Q30: In comparing acid-base neutralization reactions and redox
Q30: What is the oxidation number of chromium
Q31: Consider the following images.The reactions occurring in
Q38: Which of the following statements is incorrect?<br>A)Acid-base