Multiple Choice
Identify the oxidation half-reaction in the following group.
A) Ag(NH3) 2 + e- 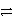 Ag + 2 NH3
Ag + 2 NH3
B) Ag + Cl- 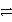 AgCl + e-
AgCl + e-
C) PbSO4(s) + 2 e- 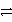 Pb(s) + SO42-
Pb(s) + SO42-
D) Ag(CN) 2 + e- 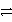 Ag + 2 CN-
Ag + 2 CN-
E) Cr2O72- + H2O 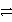 2 CrO42- + 2 H+
2 CrO42- + 2 H+
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: What role does the reducing agent play
Q5: What is the oxidation number of Cl
Q12: Which of the following is the half-reaction
Q13: Examine the two beakers shown below. <img
Q14: Which substance gets reduced in this redox
Q15: Consider the following table of relative
Q16: How many electrons are transferred in the
Q18: What is the balanced redox equation that
Q22: Which of the following substances is most
Q23: Which of the following processes is a