Multiple Choice
What is the balanced redox equation that results from the combination of the following half-reactions?
Cr3+ + 3e- 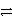
Cr and 2 Cl- 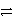
Cl2 + 2 e-
A) CrCl2+ + e- 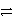 Cr + Cl2
Cr + Cl2
B) Cr3+ + 2 Cl- + e- 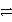 Cr + Cl2
Cr + Cl2
C) Cr3+ + 3 Cl- 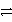 CrCl3
CrCl3
D) 2 Cr3+ + 6 Cl- + 3 e- 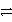 3 Cl2 + 2 Cr + 2 e-
3 Cl2 + 2 Cr + 2 e-
E) 2 Cr3+ + 6 Cl- 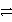 3 Cl2 + 2 Cr
3 Cl2 + 2 Cr
Correct Answer:

Verified
Correct Answer:
Verified
Q2: What role does the reducing agent play
Q5: What is the oxidation number of Cl
Q13: Examine the two beakers shown below. <img
Q14: Which substance gets reduced in this redox
Q15: Consider the following table of relative
Q16: How many electrons are transferred in the
Q17: Identify the oxidation half-reaction in the following
Q22: Which of the following substances is most
Q23: Which of the following processes is a
Q30: In comparing acid-base neutralization reactions and redox