Multiple Choice
Which of the following conforms to the Arrhenius definition of an acid?
A) H2O( 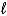 )
)
B) HBrO3(aq)
C) HCO3-(aq)
D) Al3+(aq)
E) Mg(OH) 2(s)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: Which of the following is a Brønsted-Lowry
Q25: A solution has a hydroxide ion concentration
Q27: What is the hydrogen ion concentration in
Q28: Which pair below cannot have a Brønsted-Lowry
Q29: A pH meter is used to test
Q31: The pH of a solution is 5.330.Find
Q32: What are the [H<sup>+</sup>] and [OH<sup>-</sup>] of
Q33: A solution is made by dissolving 0.0010
Q34: What is the pH of a solution
Q35: Which is the correct net ionic equation