Multiple Choice
Which is the correct net ionic equation for the reaction between HC2O4- and HPO42-? (Note: H2C2O4 is a stronger acid than H2PO4-.)
A) HC2O4- + HPO42- 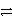 H+ + H2O42- + HPO42-
H+ + H2O42- + HPO42-
B) HC2O4- + HPO42- 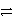 H+ + HC2O4- + PO43-
H+ + HC2O4- + PO43-
C) HC2O4- + HPO42- 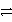 C2O42- + H2PO4-
C2O42- + H2PO4-
D) HC2O4- + HPO42- 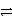 H2C2O4 + PO43-
H2C2O4 + PO43-
E) HC2O4- + HPO42- 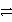 H2 + C2O42- + PO43-
H2 + C2O42- + PO43-
Correct Answer:

Verified
Correct Answer:
Verified
Q18: Which of the following properties is traditionally
Q26: A substance that can act as both
Q30: Which of the following conforms to the
Q31: The pH of a solution is 5.330.Find
Q32: What are the [H<sup>+</sup>] and [OH<sup>-</sup>] of
Q33: A solution is made by dissolving 0.0010
Q34: What is the pH of a solution
Q38: Consider the following generalized reaction. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6511/.jpg"
Q39: The hydrogen ion concentration of a solution
Q40: What is the conjugate acid of HPO<sub>4</sub><sup>2</sup><sup>-</sup>(aq)?<br>A)H<sub>3</sub>PO<sub>4</sub>(aq)<br>B)HPO<sub>4</sub><sup>2</sup><sup>-</sup>(aq)<br>C)H<sub>2</sub>PO<sub>4</sub><sup>-</sup>(aq)<br>D)PO<sub>4</sub><sup>3</sup><sup>-</sup>(aq)<br>E)H<sub>3</sub>O<sup>+</sup>(aq)