Multiple Choice
Which of the following cannot act as a Brønsted-Lowry acid?
A) HCO3-(aq)
B) HOH( 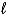 )
)
C) NH3(g)
D) CO32-(aq)
E) CH3OH( 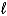 )
)
Correct Answer:

Verified
Correct Answer:
Verified
Q6: Consider a general comparison between acid-base and
Q7: Which of the following statements is incorrect
Q8: Given the following acid strengths: HF
Q9: Which of the following substances is amphoteric?<br>A)H<sub>2</sub>O(
Q12: Which of the following solutions is most
Q13: Which of the following is not capable
Q15: Identify the conjugate acid base pairs
Q30: Given the following relative base strengths,starting with
Q37: What is the conjugate base of ammonia?<br>A)NH<sub>3</sub>(g)<br>B)NH<sub>4</sub><sup>+</sup>(aq)<br>C)NH<sub>4</sub>OH(aq)<br>D)NH<sub>2</sub><sup>-</sup>(aq)<br>E)OH<sup>-</sup>(aq)
Q41: What is a Brønsted-Lowry base?<br>A)An electron pair