Multiple Choice
Which of the following is not capable of acting like a Brønsted-Lowry base?
A) H2O( 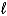 )
)
B) NH4+ ion
C) Cl- ion
D) HNO3(aq)
E) H2PO4-(aq)
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Given the following acid strengths: HF
Q9: Which of the following substances is amphoteric?<br>A)H<sub>2</sub>O(
Q10: Which of the following cannot act as
Q12: Which of the following solutions is most
Q15: Identify the conjugate acid base pairs
Q18: Which of the following is the correct
Q23: A solution is made by dissolving 12.50
Q30: Given the following relative base strengths,starting with
Q37: What is the conjugate base of ammonia?<br>A)NH<sub>3</sub>(g)<br>B)NH<sub>4</sub><sup>+</sup>(aq)<br>C)NH<sub>4</sub>OH(aq)<br>D)NH<sub>2</sub><sup>-</sup>(aq)<br>E)OH<sup>-</sup>(aq)
Q44: According to Arrhenius theory,what is an acid?<br>A)A