Multiple Choice
Consider the following image which depicts the reactants in an acid-base reaction.Atoms other than H are labeled with the element symbol. 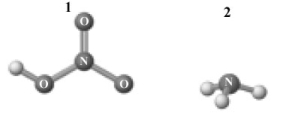 The products of this reaction are shown below.
The products of this reaction are shown below. 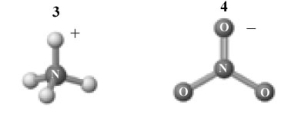
Which of the following is a correct interpretation of this reaction?
A) 1 and 2 are a conjugate acid-base pair.
B) 3 and 4are a conjugate acid-base pair.
C) 1 and 4 are a conjugate acid-base pair.
D) 2 and 4 are a conjugate acid-base pair.
E) 1 and 3 are a conjugate acid-base pair.
Correct Answer:

Verified
Correct Answer:
Verified
Q7: Which of the following statements is incorrect?<br>A)Acid-base
Q8: A water solution is considered acidic when
Q17: Which of the following is a Brønsted-Lowry
Q18: Which of the following is the correct
Q20: The hydroxide ion concentration of a solution
Q23: A solution is made by dissolving 12.50
Q23: What is the conjugate base of H<sub>3</sub>C<sub>6</sub>H<sub>5</sub>O<sub>7</sub>?<br>A)C<sub>6</sub>H<sub>5</sub>O<sub>7</sub><sup>3</sup><sup>-</sup><br>B)H<sub>2</sub>C<sub>6</sub>H<sub>5</sub>O<sub>7</sub><sup>-</sup><br>C)H<sub>3</sub>C<sub>6</sub>H<sub>5</sub>O<sub>7</sub><sup>-</sup><br>D)H<sub>4</sub>C<sub>6</sub>H<sub>5</sub>O<sub>7</sub><sup>+</sup><br>E)H<sub>3</sub>C<sub>6</sub>H<sub>5</sub>O<sub>7</sub>(OH)<sup>-</sup>
Q25: A solution has a hydroxide ion concentration
Q27: What is the hydrogen ion concentration in
Q40: In Brønsted-Lowry acid-base reactions,which of the following