Multiple Choice
Which of the following is the correct net ionic equation for the reaction between nitrous acid and hydrogen sulfide ion?
A) HNO2 + HS- 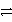 H2NO2+ + S2-
H2NO2+ + S2-
B) HNO2 + HS- 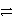 NO2- + H2S
NO2- + H2S
C) HNO2 + HSO4- 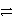 H2NO2+ + SO42-
H2NO2+ + SO42-
D) HNO2 + HSO4- 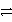 NO2- + H2SO4
NO2- + H2SO4
E) HNO3 + HSO4- 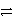 NO3- + H2SO4
NO3- + H2SO4
Correct Answer:

Verified
Correct Answer:
Verified
Q8: A water solution is considered acidic when
Q13: Which of the following is not capable
Q15: Identify the conjugate acid base pairs
Q20: The hydroxide ion concentration of a solution
Q22: Consider the following image which depicts the
Q23: A solution is made by dissolving 12.50
Q23: What is the conjugate base of H<sub>3</sub>C<sub>6</sub>H<sub>5</sub>O<sub>7</sub>?<br>A)C<sub>6</sub>H<sub>5</sub>O<sub>7</sub><sup>3</sup><sup>-</sup><br>B)H<sub>2</sub>C<sub>6</sub>H<sub>5</sub>O<sub>7</sub><sup>-</sup><br>C)H<sub>3</sub>C<sub>6</sub>H<sub>5</sub>O<sub>7</sub><sup>-</sup><br>D)H<sub>4</sub>C<sub>6</sub>H<sub>5</sub>O<sub>7</sub><sup>+</sup><br>E)H<sub>3</sub>C<sub>6</sub>H<sub>5</sub>O<sub>7</sub>(OH)<sup>-</sup>
Q30: Given the following relative base strengths,starting with
Q40: In Brønsted-Lowry acid-base reactions,which of the following
Q44: According to Arrhenius theory,what is an acid?<br>A)A