Multiple Choice
Silver nitrate and magnesium chloride solutions are mixed.What is the Per expression that allows one to convert moles of silver nitrate to moles of magnesium chloride?
A) 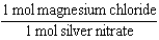
B) 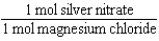
C) 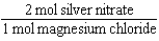
D) 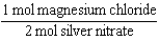
Correct Answer:

Verified
Correct Answer:
Verified
Q1: <span class="ql-formula" data-value="\Delta"><span class="katex"><span class="katex-mathml"><math xmlns="http://www.w3.org/1998/Math/MathML"><semantics><mrow><mi mathvariant="normal">Δ</mi></mrow><annotation
Q2: If the reaction N<sub>2</sub> + 3
Q3: When C<sub>2</sub>H<sub>5</sub>Cl(g)is burned in oxygen,chlorine gas
Q4: How many moles of C<sub>6</sub>H<sub>12</sub>O<sub>6</sub> are
Q6: In the reaction 2 AgI +
Q7: PCl<sub>5</sub> can be produced by the
Q8: In the reaction CaCN<sub>2</sub> + 3
Q9: Which of the following defines the percent
Q10: Cu<sub>2</sub>HgI<sub>4</sub> is prepared according to the
Q11: Aqueous HCl is added to an