Multiple Choice
H = +572 kJ for the decomposition of water by the reaction 2 H2O( 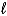 ) 2 H2(g) + O2(g) .How many grams of water can be decomposed by 5.00 *103 kJ of energy?
) 2 H2(g) + O2(g) .How many grams of water can be decomposed by 5.00 *103 kJ of energy?
A) 157 g
B) 315 g
C) 0.0629 g
D) 2.57* 107 g
E) 5.15 * 107 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: If the reaction N<sub>2</sub> + 3
Q3: When C<sub>2</sub>H<sub>5</sub>Cl(g)is burned in oxygen,chlorine gas
Q4: How many moles of C<sub>6</sub>H<sub>12</sub>O<sub>6</sub> are
Q5: Silver nitrate and magnesium chloride solutions are
Q6: In the reaction 2 AgI +
Q7: PCl<sub>5</sub> can be produced by the
Q8: In the reaction CaCN<sub>2</sub> + 3
Q9: Which of the following defines the percent
Q10: Cu<sub>2</sub>HgI<sub>4</sub> is prepared according to the
Q11: Aqueous HCl is added to an