Multiple Choice
The diagram shows the energy levels for an electron in a certain atom.Of the transitions shown, which represents the emission of a photon with the most energy? 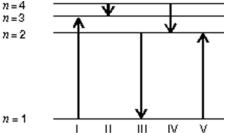
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q35: Take the potential energy of a hydrogen
Q36: A particle in a certain finite potential
Q37: The wave function for an electron in
Q38: Take the potential energy of a hydrogen
Q39: Take the potential energy of a hydrogen
Q40: An electron is trapped in an infinitely
Q41: Two one-dimensional traps have infinite potential energy
Q42: A particle is confined by finite potential
Q43: An electron is trapped in a deep
Q44: When a hydrogen atom makes the transition