Multiple Choice
The possible values for the magnetic quantum number ms of an electron in an atom:
A) depend on n
B) depend on 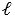
C) depend on both n and 11ef01a9_cc52_48f6_bc85_8d3f517cd313_TB6585_11
D) depend on whether or not there is an external magnetic field present
E) are 11ef0192_1f15_a623_bc85_e5393a8c0d0a_TB6585_11 1/2
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Five electrons are in a two-dimensional square
Q5: The magnitude of the spin magnetic
Q6: The number of values of the orbital
Q7: The most energetic electron in any atom
Q8: A metastable state is important for the
Q10: An electron in an atom is in
Q11: Electrons are in a two-dimensional square potential
Q12: An electron in a K shell
Q13: An electron is in a quantum state
Q14: An electron is in a quantum state