Multiple Choice
Electrons are in a two-dimensional square potential energy well with sides of length L.The potential energy is infinite at the sides and zero inside.The single-particle energies are given by 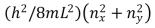 , , where nx and ny are integers.At most the number of electrons that can have energy 8(h2/8mL2) is:
, , where nx and ny are integers.At most the number of electrons that can have energy 8(h2/8mL2) is:
A) 1
B) 2
C) 3
D) 4
E) any number
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: The number of values of the orbital
Q7: The most energetic electron in any atom
Q8: A metastable state is important for the
Q9: The possible values for the magnetic quantum
Q10: An electron in an atom is in
Q12: An electron in a K shell
Q13: An electron is in a quantum state
Q14: An electron is in a quantum state
Q15: The quantum number m<sub>s</sub> is most closely
Q16: The transition shown gives rise to