Multiple Choice
Compare the two reaction coordinate diagrams below and select the answer that CORRECTLY describes their relationship. In each case, the single intermediate is the ES complex. 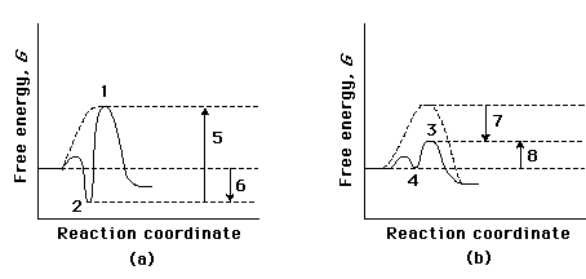
A) (a) describes a strict "lock and key" model, whereas (b) describes a transition-state complementarity model.
B) The activation energy for the catalyzed reaction is 5 in (a) and is 7 in (b) .
C) The activation energy for the uncatalyzed reaction is given by 5 + 6 in (a) and by 7 + 4 in (b) .
D) The contribution of binding energy is given by 5 in (a) and by 7 in (b) .
E) The ES complex is given by 2 in (a) and 3 in (b) .
Correct Answer:

Verified
Correct Answer:
Verified
Q49: An enzyme catalyzes a reaction at
Q50: Which enzyme uses general acid-base catalysis
Q51: The enzymatic activity of lysozyme is optimal
Q52: Michaelis and Menten assumed that the overall
Q53: A double-reciprocal plot of 1/V<sub>0</sub> versus 1/[S]
Q55: If you were attempting to design a
Q56: Blood coagulation involves:<br>A) a kinase cascade.<br>B) zymogen
Q57: Which amino acid is NOT one that
Q58: A biochemist obtains the following set
Q59: Leucine biosynthesis occurs in a multistep pathway