Not Answered
Enzymes with a kcat /Km ratio of about 108 M-1s-1 are considered to show optimal catalytic efficiency. Fumarase, which catalyzes the reversible-dehydration reaction 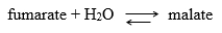 has a ratio of turnover number to the Michaelis-Menten constant, (kcat/Km) of 1.6 × 108 for the substrate fumarate and 3.6 × 107 for the substrate malate. Because the turnover number for both substrates is nearly identical, what factors might be involved that explain the different ratio for the two substrates?
has a ratio of turnover number to the Michaelis-Menten constant, (kcat/Km) of 1.6 × 108 for the substrate fumarate and 3.6 × 107 for the substrate malate. Because the turnover number for both substrates is nearly identical, what factors might be involved that explain the different ratio for the two substrates?
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Give the Michaelis-Menten equation and define each
Q2: Why is a transition-state analog not necessarily
Q4: The Mg<sup>2+</sup> metal ions involved in the
Q5: Michaelis-Menten kinetics is sometimes referred to as
Q6: V<sub>max</sub> for an enzyme-catalyzed reaction:<br>A) generally increases
Q7: In the following diagram of the first
Q8: A good transition-state analog:<br>A) binds covalently to
Q9: An enzyme follows Michaelis-Menten kinetics. Indicate (with
Q10: Write an equilibrium expression for the
Q11: An enzyme can catalyze a reaction with