Multiple Choice
How many moles of water are produced from 0.100 mol pentane,C5H₁₂? __C5H₁₂ (g) + __O₂(g) 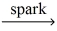 __CO₂(g) + __H₂O(g)
__CO₂(g) + __H₂O(g)
A) 0.100 mol
B) 0.500 mol
C) 0.600 mol
D) 0.800 mol
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q50: Considering the limiting reactant concept,how many moles
Q51: The reaction for photosynthesis producing glucose sugar
Q52: Starting with 0.657 g of lead(II)nitrate,a student
Q53: How many molar masses of water are
Q54: What is the mass of silver metal
Q56: What is the volume of hydrogen gas
Q57: What is the mass of aluminum metal
Q58: How many moles of hydrogen gas are
Q59: How many moles of oxygen are produced
Q60: What term refers to a type of