Multiple Choice
The reaction for photosynthesis producing glucose sugar and oxygen gas is: __CO₂(g) + __H₂O(l) 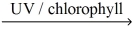 __C₆H₁₂O₆(s) + __O₂(g)
__C₆H₁₂O₆(s) + __O₂(g)
What is the volume of oxygen gas at STP produced from 2.20 g of CO₂?
A) 0.187 L
B) 1.12 L
C) 1.60 L
D) 4.32 L
E) 6.72 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q46: What is the mass of aluminum metal
Q47: Classify the following type of stoichiometry problem:
Q48: What volume of hydrogen gas reacts with
Q49: Considering the limiting reactant concept,how many moles
Q50: Considering the limiting reactant concept,how many moles
Q52: Starting with 0.657 g of lead(II)nitrate,a student
Q53: How many molar masses of water are
Q54: What is the mass of silver metal
Q55: How many moles of water are produced
Q56: What is the volume of hydrogen gas