Multiple Choice
What volume of oxygen gas reacts with 20.0 mL of hydrogen chloride? (Assume temperature and pressure remain constant. )
__ HCl(g) + __O₂(g) 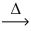 __Cl₂(g) + __H₂O(g)
__Cl₂(g) + __H₂O(g)
A) 5.00 mL
B) 10.0 mL
C) 20.0 mL
D) 40.0 mL
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q37: How many moles of oxygen gas react
Q38: How many moles of hydrogen iodide are
Q39: Starting with 1.550 g of potassium chlorate,a
Q40: What is the mass of sodium phosphate
Q41: What is the mass of iron(III)bromide (295.55
Q43: How many moles of water react with
Q44: What is the mass of insoluble calcium
Q45: What is the mass of insoluble silver
Q46: What is the mass of aluminum metal
Q47: Classify the following type of stoichiometry problem: