Multiple Choice
What volume of oxygen gas reacts with 5.00 L of propane gas,C₃H₈? (Assume temperature and pressure remain constant. )
__C₃H₈(g) + __O₂(g) 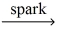 __CO₂(g) + __H₂O(g)
__CO₂(g) + __H₂O(g)
A) 1.00 L
B) 15.0 L
C) 20.0 L
D) 25.0 L
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q20: In an experiment,0.243 g of magnesium reacts
Q21: Considering the limiting reactant concept,how many moles
Q22: Which of the following steps is necessary
Q23: Considering the limiting reactant,what is the volume
Q24: How many moles of oxygen react with
Q26: What is the volume of oxygen gas
Q27: Classify the following type of stoichiometry problem:
Q28: Considering the limiting reactant,what is the volume
Q29: How many moles of oxygen gas react
Q30: Which of the following gases is blown