Multiple Choice
Considering the limiting reactant,what is the volume of NO gas produced from 30.0 L of nitrogen gas and 40.0 L of oxygen gas? (Assume constant conditions. ) N₂(g) + O₂(g) 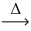 2 NO(g)
2 NO(g)
A) 30.0 L
B) 40.0 L
C) 60.0 L
D) 80.0 L
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Q18: Assuming similar conditions,how many liters of steam,H₂O,react
Q19: What is the term for the actual
Q20: In an experiment,0.243 g of magnesium reacts
Q21: Considering the limiting reactant concept,how many moles
Q22: Which of the following steps is necessary
Q24: How many moles of oxygen react with
Q25: What volume of oxygen gas reacts with
Q26: What is the volume of oxygen gas
Q27: Classify the following type of stoichiometry problem:
Q28: Considering the limiting reactant,what is the volume