Multiple Choice
Considering the limiting reactant,what mass of cobalt(III) sulfide (214.07 g/mol) is produced from 0.750 g of cobalt and 0.350 g of sulfur? 2 Co(s) + 3 S(s) 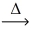 CO₂S3(s)
CO₂S3(s)
A) 0.779 g
B) 1.36 g
C) 2.34 g
D) 2.72 g
E) 5.45 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q75: What principle states that equal volumes of
Q76: Which of the following are in the
Q77: What law states the total volume of
Q78: What is the mass of aluminum metal
Q79: The decomposition of 1.500 g of potassium
Q81: What is the mass of aluminum oxide
Q82: Considering the limiting reactant,what is the mass
Q83: What volume of oxygen gas reacts with
Q84: How many moles of water react with
Q85: What is the mass of iron produced