Multiple Choice
What is the mass of iron produced from 225 g of iron(III) oxide (159.70 g/mol) ? __Fe₂O₃(l) + __CO(g) 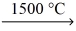 __Fe(l) + __CO₂(g)
__Fe(l) + __CO₂(g)
A) 39.3 g
B) 78.7 g
C) 157 g
D) 322 g
E) 1290 g
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q80: Considering the limiting reactant,what mass of cobalt(III)sulfide
Q81: What is the mass of aluminum oxide
Q82: Considering the limiting reactant,what is the mass
Q83: What volume of oxygen gas reacts with
Q84: How many moles of water react with
Q86: Assuming similar conditions,how many liters of carbon
Q87: What is the volume of oxygen gas
Q88: How many moles of steam,H₂O,react with 1
Q89: Considering the limiting reactant,how many moles of
Q90: Which of the following is not in