Multiple Choice
What volume of carbon monoxide gas at STP reacts to produce 759 g of iron? __Fe₂O₃(l) + __CO(g) 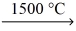 __Fe(l) + __CO₂(g)
__Fe(l) + __CO₂(g)
A) 152 L
B) 203 L
C) 305 L
D) 457 L
E) 914 L
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q110: How many moles of nitrogen dioxide gas
Q111: What volume of methane gas,CH₄,reacts to give
Q112: How many moles of oxygen gas react
Q113: In an experiment,1.201 g of charcoal reacts
Q114: Considering the limiting reactant,what is the volume
Q116: Considering the limiting reactant,what is the volume
Q117: What is the mass of mercuric oxide
Q118: How many moles of water are produced
Q119: How many molar masses of CO₂ gas
Q120: How many moles of iodine vapor react