Multiple Choice
How many moles of nitrogen dioxide gas are produced from 1.00mol NO? __NO(g) + __O₂(g) 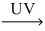 __NO₂(g)
__NO₂(g)
A) 0.250 mol
B) 0.500 mol
C) 1.00 mol
D) 2.00 mol
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q105: What is the term for the amount
Q106: In an experiment,5.585 g of iron metal
Q107: Considering the limiting reactant concept,how many moles
Q108: How many moles of chlorine gas react
Q109: How many moles of hydrogen gas are
Q111: What volume of methane gas,CH₄,reacts to give
Q112: How many moles of oxygen gas react
Q113: In an experiment,1.201 g of charcoal reacts
Q114: Considering the limiting reactant,what is the volume
Q115: What volume of carbon monoxide gas at