Multiple Choice
For the following transformation how many H atoms are added or lost? 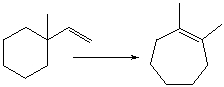
A) Added one
B) Added two
C) Lost one
D) Lost two
E) No change
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q4: Which of the following is the correct
Q6: What is the formal charge on the
Q7: Which of the following is the correct
Q8: The lone pair on nitrogen in the
Q10: For the following compound identify the lone
Q11: Which of the following compounds contain an
Q12: How many total lone pairs of electrons
Q13: Which of the following is the correct
Q14: For the following compound identify the lone
Q47: Draw a bond-line structure for CH<sub>3</sub>CH<sub>2</sub>O(CH<sub>2</sub>)<sub>2</sub>CH(CH<sub>3</sub>)<sub>2</sub>.