Multiple Choice
Which of the following is the correct bond-line structure for (CH3) 2CHCH2CH3? 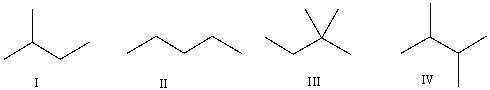
A) I
B) II
C) III
D) IV
E) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: Which of the following violates the rules
Q3: What is the relationship between the following
Q4: Which of the following is the correct
Q6: What is the formal charge on the
Q8: The lone pair on nitrogen in the
Q9: For the following transformation how many H
Q10: For the following compound identify the lone
Q11: Which of the following compounds contain an
Q12: How many total lone pairs of electrons
Q47: Draw a bond-line structure for CH<sub>3</sub>CH<sub>2</sub>O(CH<sub>2</sub>)<sub>2</sub>CH(CH<sub>3</sub>)<sub>2</sub>.