Multiple Choice
Given the bond dissociation energies below (in kcal/mol) , estimate the ΔH° for the propagation step 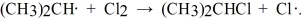
CH3CH2CH2-H 98
(CH3) 2CH-H 95
Cl-Cl 58
H-Cl 103
CH3CH2CH2-Cl 81
(CH3) 2CH-Cl 80
A) -22 kcal/mol
B) +22 kcal/mol
C) -40 kcal/mol
D) +45 kcal/mol
Correct Answer:

Verified
Correct Answer:
Verified
Q45: Provide the structure of the transition state
Q46: The following reaction occurs readily: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6198/.jpg"
Q53: In an exothermic reaction, are stronger bonds
Q53: For a given reaction, if ΔG° is
Q54: Rank the free radicals (I-III) shown below
Q55: Do you expect the initiation step in
Q56: What accounts for the relatively high stability
Q63: Which of the halogens below undergoes free
Q119: What term describes the highest-energy structure in
Q121: Given that tertiary H atoms react with