Short Answer
The following reaction occurs readily: 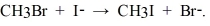 Experimentally one finds that if the concentration of I- is doubled, the rate doubles. Also if the concentration of CH3Br is halved, the rate is halved. What is the rate equation for this reaction?
Experimentally one finds that if the concentration of I- is doubled, the rate doubles. Also if the concentration of CH3Br is halved, the rate is halved. What is the rate equation for this reaction?
Correct Answer:

Verified
Correct Answer:
Verified
Q42: Do you expect the initiation step in
Q44: Remove an H+ from the following structure
Q45: Provide the structure of the transition state
Q48: Provide the major organic product that results
Q51: Given the bond dissociation energies below (in
Q63: Which of the halogens below undergoes free
Q71: When 1,1,3,3-tetramethylcyclobutane is brominated at 125°C, the
Q93: How do alkyl substituents stabilize a carbocationic
Q119: What term describes the highest-energy structure in
Q121: Given that tertiary H atoms react with