Multiple Choice
Which one of the following Lewis structures is definitely incorrect?
A) 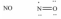
B) 
C) 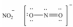
D) 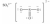
E) 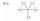
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q5: Identify the correct equation to calculate the
Q15: How many dots does the Lewis dot
Q24: Select the element whose Lewis symbol is
Q25: Ionic compounds may carry a net positive
Q48: Describe, with appropriate explanations, the key factors
Q54: Select the compound with the highest (i.e.,
Q61: Which statement is false concerning ionic bonds
Q65: Which of these compounds is most likely
Q84: A double bond cannot exist between a
Q94: Which of these ionic solids would have