Multiple Choice
In the following Lewis structure for ClO3F,chlorine has a formal charge of ____ and an oxidation number of ____. 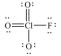
A) 7,7
B) 7,-1
C) 1,1
D) 1,-1
E) 1,7
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Which of the following represents the most
Q12: In which one of the following structures
Q15: How many dots does the Lewis dot
Q20: How many single bonds are bonded to
Q29: What is the formal charge on the
Q41: In which of the following species does
Q45: The Lewis dot symbol consists of the
Q76: How many dots does the Lewis dot
Q87: A triple bond arises when two atoms
Q129: In the Lewis structure of the iodate