Multiple Choice
In which one of the following structures does the central atom have a formal charge of +2?
A) 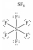
B) 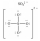
C) 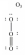
D) 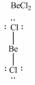
E) 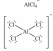
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: Which of the following represents the most
Q10: In the following Lewis structure for ClO<sub>3</sub>F,chlorine
Q15: How many dots does the Lewis dot
Q20: How many single bonds are bonded to
Q25: Ionic compounds may carry a net positive
Q29: What is the formal charge on the
Q41: In which of the following species does
Q45: The Lewis dot symbol consists of the
Q48: Describe, with appropriate explanations, the key factors
Q87: A triple bond arises when two atoms