Exam 7: Alkyl Halides and Nucleophilic Substitution
Exam 1: Structure and Bonding77 Questions
Exam 2: Acids and Bases59 Questions
Exam 3: Introduction to Organic Molecules and Functional Groups56 Questions
Exam 4: Alkanes64 Questions
Exam 5: Stereochemistry76 Questions
Exam 6: Understanding Organic Reactions53 Questions
Exam 7: Alkyl Halides and Nucleophilic Substitution73 Questions
Exam 8: Alkyl Halides and Elimination Reactions52 Questions
Exam 9: Alcohols,ethers,and Related Compounds60 Questions
Exam 10: Alkenes and Addition Reactions54 Questions
Exam 11: Alkynes and Synthesis51 Questions
Exam 12: Oxidation and Reduction51 Questions
Exam 13: Radical Reactions51 Questions
Exam 14: Conjugation, resonance, and Dienes53 Questions
Exam 15: Benzene and Aromatic Compounds47 Questions
Exam 16: Reactions of Aromatic Compounds60 Questions
Exam 17: Introduction to Carbonyl Chemistry; Organometallic Reagents; Oxidation and Reduction59 Questions
Exam 18: Aldehydes and Ketones - Nucleophilic Addition52 Questions
Exam 19: Carboxylic Acids and the Acidity of the O-H Bond53 Questions
Exam 20: Carboxylic Acids and Their Derivatives - Nucleophilic Acyl Substitution50 Questions
Exam 21: Substitution Reactions of Carbonyl Compounds at the a Carbon47 Questions
Exam 22: Carbonyl Condensation Reactions51 Questions
Exam 23: Amines65 Questions
Exam 24: Carbon-Carbon Bond-Forming Reactions in Organic Synthesis47 Questions
Exam 25: Pericyclic Reactions62 Questions
Exam 26: Carbohydrates50 Questions
Exam 27: Amino Acids and Proteins46 Questions
Exam 28: Synthetic Polymers45 Questions
Exam 29: Lipids45 Questions
Exam 30: Molecular Ion Peaks and Fragmentation Patterns19 Questions
Exam 31: Analyzing Molecular Motion and Infrared Spectroscopy37 Questions
Exam 32: Organic Spectroscopy Concepts51 Questions
Select questions type
Which of the following rate laws describes the kinetics of an SN1 reaction?
(Multiple Choice)
5.0/5  (29)
(29)
Which of the following is not a characteristic of an SN1 reaction?
(Multiple Choice)
4.7/5  (29)
(29)
Which of the following statements about the reactions of alkyl halides is true?
(Multiple Choice)
4.7/5  (46)
(46)
Which of the following structures have the correct common name? 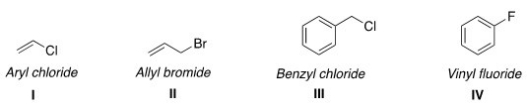
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following statements about the SN2 mechanism for nucleophilic substitution reactions is true?
(Multiple Choice)
4.7/5  (29)
(29)
The reaction of tert-butyl bromide,(CH3)3CBr,with ethanol affords the substitution product tert-butyl ethyl ether,(CH3)3COCH2CH3,in acidic conditions.What would happen to the rate of the reaction if the concentration of ethanol was doubled?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following anions is the most nucleophilic in polar aprotic solvents?
(Multiple Choice)
4.8/5  (41)
(41)
Which compound is most likely to follow second-order kinetics in a substitution reaction?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following statements about an SN1 reaction mechanism is true?
(Multiple Choice)
4.7/5  (34)
(34)
Rank the following carbocations in order of decreasing stability,putting the most stable first. 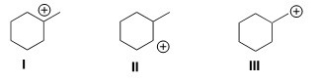
(Multiple Choice)
4.8/5  (30)
(30)
What is the product of the nucleophilic substitution reaction shown below? 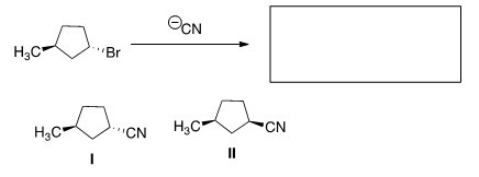
(Multiple Choice)
4.8/5  (30)
(30)
The reaction of bromoethane with sodium acetate affords the substitution product methyl acetate.What is the effect of doubling the concentration of sodium acetate on the rate of the reaction?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the following statements about carbocation stability is true?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following statements about the Hammond postulate is not true?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following solvents is not a polar protic solvent? 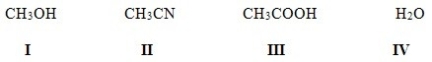
(Multiple Choice)
4.8/5  (32)
(32)
The reaction of 1-bromobutane with sodium hydroxide affords the substitution product 1-butanol.What would happen to the rate of the reaction if the concentration of both 1-bromobutane and sodium hydroxide were doubled?
(Multiple Choice)
4.8/5  (32)
(32)
Showing 41 - 60 of 73
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)