Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
Which of the following statements is true concerning the electrochemical cell Zn(s) | Zn2+(aq, 1.0 M) || Ca2+(aq, 1.0 M) | Ca(s) The standard reduction potentials are given follows:
Zn2+(aq) + 2 e− → Zn(s); E° = -0.76 V
Ca2+(aq) + 2 e− → Ca(s); E° = -2.87 V
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following statements is true concerning the voltaic cell shown below? 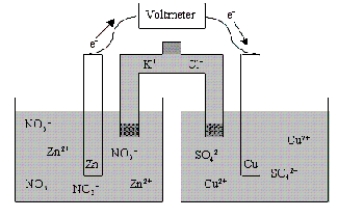
(Multiple Choice)
4.8/5  (47)
(47)
Which of the following is true for a product-favored reaction at equilibrium?
(Multiple Choice)
4.8/5  (29)
(29)
Write a balanced half-reaction for the reduction of hydrogen peroxide to water in an acidic solution.
(Multiple Choice)
4.8/5  (32)
(32)
For the following cell reaction, the standard cell potential is 1.34 V. To determine the cell potential at nonstandard conditions, what is the value that should be used for n in the Nernst equation?
(Multiple Choice)
4.7/5  (35)
(35)
Calculate ΔrG° for the disproportionation reaction of copper(I) ion (Cu+) at 25 °C. 2 Cu+(aq) → Cu2+(aq) + Cu(s)
The standard reduction potentials are as follows:
Cu+(aq) + e- → Cu(s)
E° = +0.518 V
Cu2+(aq) + 2 e- → Cu(s)
E° = +0.337 V
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following statements concerning voltaic cells is not true?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following is the cell notation for a cell in which the hydrogen electrode is the anode and the cathode half-reaction is
Co3+(aq) + e− → Co2+(aq)?
(Multiple Choice)
4.9/5  (30)
(30)
The standard reduction potentials are as follows: Cr3+(aq) + 3 e- → Cr(s); E° = -0.74 V
Fe2+(aq) + 2 e- → Fe(s); E° = -0.41 V
Calculate the standard Gibbs free energy change for the following reaction.
2 Cr(s) + 3 Fe2+ → 3 Fe(s) + 2 Cr3+(aq)
(Multiple Choice)
4.8/5  (35)
(35)
Balance the following half-reaction occurring in an acidic solution.
NO3-(aq) → NO(aq)
(Multiple Choice)
4.9/5  (37)
(37)
Batteries used in watches contain mercury(II) oxide. As the current flows, mercury(II) oxide is reduced to mercury according to the following reaction: HgO(s) + H2O( ) + 2 e- ? Hg( ) + 2 OH-(aq)
If 2.3 × 10-5 amperes flows continuously for 1200 days, calculate the mass of mercury, Hg( ), produced.
(Multiple Choice)
4.9/5  (40)
(40)
The standard cell potential of the given electrochemical cell is 0.19 V. Pt | Sn4+(aq, 1.0 M), Sn2+(aq, 1.0 M) || Cu2+(aq, 0.200 M) | Cu
Which of the following factors will increase the measured cell potential of the given electrochemical cell?
(Multiple Choice)
4.8/5  (37)
(37)
According to the cell notation below, which of the following species is undergoing reduction?
Ni | Ni2+(aq) || Mn2+(aq) | MnO2(s) | Pt(s)
(Multiple Choice)
4.9/5  (40)
(40)
What half-reaction occurs at the cathode during the electrolysis of molten potassium bromide?
(Multiple Choice)
4.9/5  (38)
(38)
If ?rG° for the following reaction is -22.2 kJ/mol-rxn, calculate for the following reaction: Cu2+(aq) + 2 Ag(s) + 2 Cl-(aq) ? Cu(s) + 2 AgCl(s)
(Multiple Choice)
4.7/5  (39)
(39)
The cell potential of the following electrochemical cell is determined by using an unspecified concentration of acid. Calculate the pH of the acid solution, given that the measured cell potential is -0.431 V and the anode reduction potential (E°) is 0.222 V at 25 °C.
Ag(s) | AgCl(s) | Cl−(aq, 1.0 M) || H+(aq) | H2(g, 1.0 atm) | Pt(s)
(Multiple Choice)
4.9/5  (39)
(39)
Calculate Ecell for the following electrochemical cell at 25 °C Pt(s) | H2(g, 1.00 atm) | H+(aq, 1.00 M) || Sn2+(aq, 0.350 M), Sn4+(aq, 0.020 M) | Pt(s)
The standard reduction potentials are as follows:
Sn4+(aq) + 2 e- → Sn2+(s)
E° = +0.15 V
2 H+(aq) + 2 e- → H2(g)
E° = 0.00 V
(Multiple Choice)
4.8/5  (41)
(41)
The use of electrical energy to produce chemical change is known as _____. An example of this process is the reduction of sodium chloride, NaCl( ), to produce solid sodium.
(Short Answer)
4.8/5  (42)
(42)
Which of the following statements is true for the following reaction, assuming the given reaction proceeds in the forward direction?
Fe3+(aq) + Co(s) → Fe2+(aq) + Co2+(aq)
(Multiple Choice)
4.9/5  (35)
(35)
Which of the following equations represents the Nernst equation?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 21 - 40 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)