Exam 8: The Structure of Atoms and Periodic Trends
Exam 1: Basic Concepts of Chemistry40 Questions
Exam 2: Lets Review: the Tools of Quantitative Chemistry73 Questions
Exam 3: Atoms, Molecules, and Ions104 Questions
Exam 4: Chemical Reactions72 Questions
Exam 5: Stoichiometry: Quantitative Information About Chemical Reactions77 Questions
Exam 6: Principles of Chemical Reactivity: Energy and Chemical Reactions69 Questions
Exam 7: The Structure of Atoms65 Questions
Exam 8: The Structure of Atoms and Periodic Trends80 Questions
Exam 9: Bonding and Molecular Structure93 Questions
Exam 10: Bonding and Molecular Structure Orbital Hybridization and Molecular Orbitals66 Questions
Exam 11: Gases and Their Properties89 Questions
Exam 12: Intermolecular Forces and Liquids64 Questions
Exam 13: The Solid State67 Questions
Exam 14: Solutions and Their Behavior80 Questions
Exam 15: Chemical Kinetics: the Rates of Chemical Reactions74 Questions
Exam 16: Principles of Chemical Reactivity: Equilibria75 Questions
Exam 17: Principles of Chemical Reactivity: the Chemistry of Acids and Bases97 Questions
Exam 18: Principles of Chemical Reactivity: Other Aspects of Aqueous Equilibria87 Questions
Exam 19: Principles of Chemical Reactivity: Entropy and Free Energy70 Questions
Exam 20: Principles of Chemical Reactivity: Electron Transfer Reactions83 Questions
Exam 21: Environmental Chemistry: Earths Environment, Energy, and Sustainability51 Questions
Exam 22: The Chemistry of the Main Group Elements81 Questions
Exam 23: The Chemistry of the Transition Elements80 Questions
Exam 24: Carbon: Not Just Another Element88 Questions
Exam 25: Biochemistry40 Questions
Exam 26: Nuclear Chemistry189 Questions
Select questions type
All of the following ground-state electron configurations are correct except
(Multiple Choice)
4.8/5  (44)
(44)
Which element has the following ground state electron configuration? 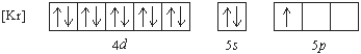
(Multiple Choice)
4.8/5  (44)
(44)
________ rule states that the most stable arrangement of electrons is that which contains the maximum number of unpaired electrons, all with the same spin direction.
(Short Answer)
4.8/5  (33)
(33)
An atom of which of the following elements has the smallest ionization energy?
(Multiple Choice)
4.8/5  (32)
(32)
Rank the following atoms in order decreasing atomic radii: Be, Be, Be, B.
(Multiple Choice)
4.8/5  (38)
(38)
The ground-state electron configuration of a Ni2+ ion is 1s22s22p63s23p63d8 . Therefore, Ni2+ is
(Multiple Choice)
4.8/5  (33)
(33)
Which of the given ions have the same ground state electron configuration: S2-, N3-, Mg2+, and Br-?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following atoms is diamagnetic in its ground state?
(Multiple Choice)
4.9/5  (36)
(36)
An atom of which of the following elements has the most negative electron affinity?
(Multiple Choice)
4.8/5  (29)
(29)
The change in energy for the following reaction is referred to as the ____ for boron.
B(g) + e- → B-(g)
(Multiple Choice)
4.8/5  (45)
(45)
Place the following ions in order from smallest to largest ionic radii:
K+, Na+, Mg2+, and Al3+.
(Multiple Choice)
4.9/5  (37)
(37)
What 2+ ion has the following ground state electron configuration? 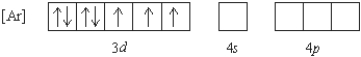
(Multiple Choice)
4.9/5  (32)
(32)
An atom of which of the following elements has the smallest atomic radius?
(Multiple Choice)
4.9/5  (31)
(31)
As one moves horizontally from left to right across a period, the effective ________ charge increases, resulting in decreasing atomic radii.
(Short Answer)
4.8/5  (30)
(30)
Which of the following statements concerning the Pauli exclusion principle is/are CORRECT?
1. If two electrons occupy the same orbital they must have opposite spins.
2. No two electrons in an atom can have the same four quantum numbers.
3. Electrons with opposing spins are attracted to each other.
(Multiple Choice)
4.7/5  (43)
(43)
Which of the following ions has the given ground state electron configuration? 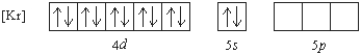
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following statements concerning the Pauli exclusion principle is/are CORRECT?
1.If two electrons occupy the same orbital they must have opposite spins.
2.No two electrons in an atom can have the same four quantum numbers.
3.Electrons with opposing spins are attracted to each other.
(Multiple Choice)
4.8/5  (34)
(34)
Showing 21 - 40 of 80
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)