Exam 4: Atomic Physics and Spectra
Exam 1: Discovering the Night Sky374 Questions
Exam 2: Gravitation and the Motion of the Planets356 Questions
Exam 3: Light and Telescopes275 Questions
Exam 4: Atomic Physics and Spectra223 Questions
Exam 5: Exoplanets and the Formation of Planetary Systems98 Questions
Exam 6: Formation of the Solar System121 Questions
Exam 7: Earth and the Moon305 Questions
Exam 8: The Other Terrestrial Planets265 Questions
Exam 9: The Outer Planets360 Questions
Exam 10: Vagabonds of the Solar System198 Questions
Exam 11: The Sun: Our Extraordinary Star248 Questions
Exam 12: Characterizing Stars254 Questions
Exam 13: The Lives of Stars From Birth Through Middle Age325 Questions
Exam 14: The Death of Stars235 Questions
Exam 15: Black Holes: Matters of Gravity178 Questions
Exam 16: The Milky Way Galaxy157 Questions
Exam 17: Galaxies207 Questions
Exam 18: Quasars and Other Active Galaxies118 Questions
Exam 19: Cosmology217 Questions
Exam 20: Astrobiology71 Questions
Select questions type
The value of max in the spectrum given off by a heated blackbody depends on the object's
(Multiple Choice)
4.7/5  (45)
(45)
One isotope of iron has an atomic number of 26. How many protons are there in its nucleus?
(Multiple Choice)
4.8/5  (36)
(36)
The temperature scale most often used by scientists is the
(Multiple Choice)
4.7/5  (34)
(34)
The temperature at the top of the clouds on Jupiter is about 165 K. In degrees Celsius, this temperature is
(Multiple Choice)
4.9/5  (39)
(39)
The series of spectral absorption lines in the infrared part of the spectrum that result from atomic transitions in hydrogen atoms in which electrons are lifted from the n = 3 level to all other atomic energy levels is known as
(Multiple Choice)
4.8/5  (45)
(45)
The wavelength at which the spectrum emitted from a blackbody is most intense is proportional to what power of the blackbody's Kelvin temperature?
(Multiple Choice)
4.9/5  (39)
(39)
Compared to the mass of an electron, the mass of a proton is
(Multiple Choice)
4.8/5  (37)
(37)
When an atom of the radioactive carbon isotope 14C decays into 14N (nitrogen), what happens in the nucleus of the atom?
(Multiple Choice)
4.9/5  (36)
(36)
What is the total number of protons and neutrons in the nucleus of an atom of the fissionable isotope of uranium used in nuclear weapons, 235U, which has an atomic number of 92?
(Multiple Choice)
4.8/5  (37)
(37)
In a simple atom, the electrons making transitions between the outermost energy levels and the innermost energy levels give rise to photons with the
(Multiple Choice)
4.7/5  (45)
(45)
What will be the peak wavelength of electromagnetic radiation emitted by a piece of iron that is just melting at a temperature of 1808 K? (Use Wien's law, which relates the peak wavelength max emitted by a body to its temperature T, and see Figure 4-2) (1 m = 10-6 m). 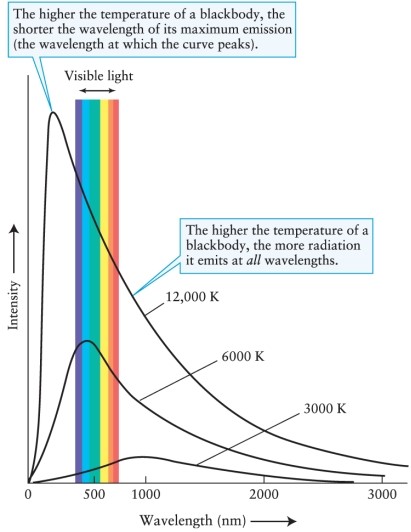
(Multiple Choice)
4.9/5  (37)
(37)
A small particle of interplanetary material is heated by friction from a temperature of 400 K to 4000 K as it falls into the atmosphere of Earth and produces a meteor or a shooting star in the sky. If this object behaves like a perfect blackbody over this short time, how will its emitted radiation change as it is heated?
(Multiple Choice)
4.9/5  (26)
(26)
The Doppler effect is the change in the wavelength of light caused by the source
(Multiple Choice)
4.7/5  (43)
(43)
Which of these items is considered to be the BEST one to use in a spectrograph to disperse light into its spectrum of colors?
(Multiple Choice)
4.9/5  (29)
(29)
What evidence exists that the Sun contains the element iron?
(Multiple Choice)
4.8/5  (30)
(30)
If the human eye has evolved over time so that its peak wavelength sensitivity is about 0.5 m (1 m = 10-6 m), what will be the temperature of a blackbody to which the eye will be MOST sensitive?
(Multiple Choice)
4.8/5  (36)
(36)
Which very significant fact concerning the spectra produced by hot gases, such as elements heated on the solar surface (Fraunhofer, with the solar spectrum) or in a flame (Bunsen and Kirchhoff, with laboratory spectra), was discovered in the 1800s?
(Multiple Choice)
4.8/5  (24)
(24)
How many electrons surround the nucleus of a neutral atom of the isotope 18O of oxygen? Oxygen has an atomic number of 8.
(Multiple Choice)
4.9/5  (35)
(35)
An astronomer plots the blackbody curve for an object at 8000 K and then raises the temperature of the object to 12,000 K and plots the blackbody curve again. These curves
(Multiple Choice)
4.7/5  (33)
(33)
Showing 181 - 200 of 223
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)