Exam 4: Atomic Physics and Spectra
Exam 1: Discovering the Night Sky374 Questions
Exam 2: Gravitation and the Motion of the Planets356 Questions
Exam 3: Light and Telescopes275 Questions
Exam 4: Atomic Physics and Spectra223 Questions
Exam 5: Exoplanets and the Formation of Planetary Systems98 Questions
Exam 6: Formation of the Solar System121 Questions
Exam 7: Earth and the Moon305 Questions
Exam 8: The Other Terrestrial Planets265 Questions
Exam 9: The Outer Planets360 Questions
Exam 10: Vagabonds of the Solar System198 Questions
Exam 11: The Sun: Our Extraordinary Star248 Questions
Exam 12: Characterizing Stars254 Questions
Exam 13: The Lives of Stars From Birth Through Middle Age325 Questions
Exam 14: The Death of Stars235 Questions
Exam 15: Black Holes: Matters of Gravity178 Questions
Exam 16: The Milky Way Galaxy157 Questions
Exam 17: Galaxies207 Questions
Exam 18: Quasars and Other Active Galaxies118 Questions
Exam 19: Cosmology217 Questions
Exam 20: Astrobiology71 Questions
Select questions type
Stars A and B have the same radius, but the spectrum of star A peaks at a wavelength of 500 nm, whereas star B's spectrum peaks at 1000 nm. What is the ratio of the luminosity of star A to the luminosity of star B?
(Multiple Choice)
4.8/5  (43)
(43)
As a new star evolves from COOL dust and gas to a HOT star, the peak wavelength of its spectrum of emitted electromagnetic radiation could
(Multiple Choice)
4.7/5  (43)
(43)
A hydrogen atom in a low-density, hot gas gives off what type of spectrum?
(Multiple Choice)
4.7/5  (39)
(39)
When a blackbody is heated to a temperature T, its total energy flux per second per unit area F at all wavelengths (where is a constant) is given by
(Multiple Choice)
4.8/5  (38)
(38)
Emission spectra from interstellar gas clouds glow with a variety of colors. Red usually indicates hydrogen, and green is usually a characteristic of
(Multiple Choice)
4.9/5  (28)
(28)
If one neutron is added to a nucleus of an isotope of carbon, 12C, in a particular nuclear reaction, the result will be
(Multiple Choice)
4.8/5  (26)
(26)
The temperature of the surface of the Sun is 5800 K. What would be the surface temperature of a star that emits twice the energy flux (watts per square meter) that the Sun emits?
(Multiple Choice)
4.7/5  (31)
(31)
Hydrogen gas is heated to the point at which there are electrons at atomic energy levels up to the n = 3 level. When electrons return to the ground state, what possible emission lines from which spectral sequences will result? See Figure 4-11. 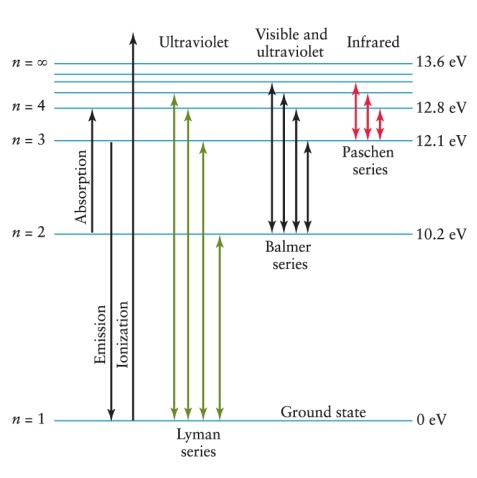
(Multiple Choice)
4.7/5  (30)
(30)
A source emitting waves of constant wavelength approaches an observer, then passes the observer, and recedes into the distance-all at a constant speed. The wavelength of the waves the observer measures from this source
(Multiple Choice)
4.8/5  (38)
(38)
Considering the oxygen isotopes 15O and 16O, which of these properties is/are very nearly the same for both isotopes?
(Multiple Choice)
4.8/5  (36)
(36)
Light that originates in hydrogen atoms in which electrons have jumped from high levels to the level n = 2 will be part of which series of spectral lines?
(Multiple Choice)
4.8/5  (21)
(21)
The overall charge of a singly ionized neon atom Ne, whose position in the periodic table (or atomic number) is 10, in units of electron charge, is
(Multiple Choice)
4.8/5  (41)
(41)
Two stars, A and B, have the same temperature, but star A is 4 times larger in diameter than star B. Which of these statements about these stars is true?
(Multiple Choice)
4.8/5  (43)
(43)
The sequence of ultraviolet emission lines emitted by hot hydrogen gas is known as the _____ series.
(Multiple Choice)
5.0/5  (29)
(29)
The isotope 20Ne has an atomic number of 10. The nucleus of this isotope contains _____ protons and _____ neutrons.
(Multiple Choice)
4.9/5  (47)
(47)
The physical force that holds the components of an atom together is the
(Multiple Choice)
4.8/5  (35)
(35)
Showing 21 - 40 of 223
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)