Exam 4: Atomic Physics and Spectra
Exam 1: Discovering the Night Sky374 Questions
Exam 2: Gravitation and the Motion of the Planets356 Questions
Exam 3: Light and Telescopes275 Questions
Exam 4: Atomic Physics and Spectra223 Questions
Exam 5: Exoplanets and the Formation of Planetary Systems98 Questions
Exam 6: Formation of the Solar System121 Questions
Exam 7: Earth and the Moon305 Questions
Exam 8: The Other Terrestrial Planets265 Questions
Exam 9: The Outer Planets360 Questions
Exam 10: Vagabonds of the Solar System198 Questions
Exam 11: The Sun: Our Extraordinary Star248 Questions
Exam 12: Characterizing Stars254 Questions
Exam 13: The Lives of Stars From Birth Through Middle Age325 Questions
Exam 14: The Death of Stars235 Questions
Exam 15: Black Holes: Matters of Gravity178 Questions
Exam 16: The Milky Way Galaxy157 Questions
Exam 17: Galaxies207 Questions
Exam 18: Quasars and Other Active Galaxies118 Questions
Exam 19: Cosmology217 Questions
Exam 20: Astrobiology71 Questions
Select questions type
How many electrons orbit the nucleus of a singly ionized oxygen atom? The atomic number of oxygen is 8.
(Multiple Choice)
4.9/5  (28)
(28)
Isotopes of a particular element in the periodic table have the same number of _____ in the nucleus.
(Multiple Choice)
4.9/5  (37)
(37)
Which of these is NOT one of the four fundamental forces in nature?
(Multiple Choice)
4.9/5  (33)
(33)
As a newly formed star continues to contract, its temperature increases, while the chemical nature of the gas does not change. Which of these could happen to the peak wavelength of its emitted radiation?
(Multiple Choice)
4.9/5  (40)
(40)
If all stars are considered to be perfect blackbodies, then which of these relationships should hold regarding the energy flux, or energy emitted per unit area, from stars?
(Multiple Choice)
4.8/5  (32)
(32)
A scientist reports that his measurement of the temperature of the surface of a newly discovered planet is -20 K. What conclusion can be drawn from this report?
(Multiple Choice)
4.8/5  (31)
(31)
Carbon-14 (14C) has a half-life of approximately 6000 years. A researcher investigating a piece of ancient wood determines that there is only 1/4 as much 14C present in the wood now as there was when the tree that the wood came from was alive. How old is the piece of wood?
(Multiple Choice)
4.8/5  (28)
(28)
When a solid body (or a dense gas such as a star) cools from a temperature of several thousand degrees, the "color" or wavelength of maximum emission of radiation will
(Multiple Choice)
4.8/5  (41)
(41)
Where and by what technique was the element helium first discovered?
(Multiple Choice)
4.7/5  (40)
(40)
An electron is in the n = 3 energy level in a hydrogen atom. What can be said about the spectral series in which it can participate by making a single atomic transition?
(Multiple Choice)
4.8/5  (30)
(30)
An electron is in the n = 3 energy level in a hydrogen atom. To ionize this atom, it is necessary for the electron to gain a minimum of how much energy? See Figure 4-11. 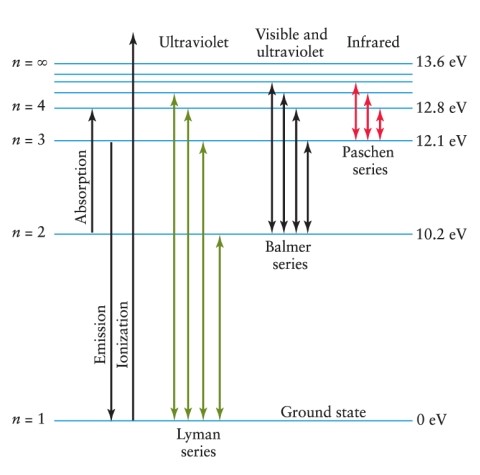
(Multiple Choice)
4.9/5  (30)
(30)
Radiant energy shines on a blackbody, raising its temperature and causing it to emit radiation. The spectrum of radiation it emits depends on
(Multiple Choice)
4.7/5  (36)
(36)
The important breakthrough in theoretical physics that was first suggested by Planck to explain the shape of the spectrum of a hot body was the
(Multiple Choice)
4.9/5  (25)
(25)
Of the four combinations of particles that form the nuclei of atoms, which one does NOT make up the nucleus of an isotope of the same element as the other three?
(Multiple Choice)
4.8/5  (35)
(35)
How much heavier is a typical hydrogen atom than a proton?
(Multiple Choice)
4.8/5  (28)
(28)
Atoms in a low-density, hot gas (e.g., in a fluorescent lamp or a neon tube) emit a spectrum that is
(Multiple Choice)
4.7/5  (42)
(42)
An astronomer observes two stars of the same surface temperature, but one has twice the diameter of the other. How many times larger is the energy production rate per unit surface area from the larger star compared with that from the smaller star?
(Multiple Choice)
4.8/5  (28)
(28)
Showing 121 - 140 of 223
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)