Exam 3: Structure and Properties of Ionic and Covalent Compounds
Exam 1: Chemistry:methods and Measurement94 Questions
Exam 2: The Structure of the Atom and the Periodic Table109 Questions
Exam 3: Structure and Properties of Ionic and Covalent Compounds91 Questions
Exam 4: Calculations, Chemical Changes, and the Chemical Equation85 Questions
Exam 5: States of Matter- Gases, Liquids, and Solids75 Questions
Exam 6: Solutions67 Questions
Exam 7: Energy, Rate and Equilibrium61 Questions
Exam 8: Acids and Bases59 Questions
Exam 9: The Nucleus, Radioactivity, and Nuclear Medicine82 Questions
Exam 10: An Introduction to Organic Chemistry- the Saturated Hydrocarbons94 Questions
Exam 11: The Unsaturated Hydrocarbons- Alkenes, Alkynes, and Aromatics65 Questions
Exam 12: Alcohols, Phenols, Thiols, and Ethers65 Questions
Exam 13: Aldehydes and Ketones55 Questions
Exam 14: Carboxylic Acids and Carboxylic Acid Derivatives80 Questions
Exam 15: Amines and Amides80 Questions
Exam 16: Carbohydrates74 Questions
Exam 17: Lipids and Their Functions in Biochemical Systems80 Questions
Exam 18: Protein Structure and Function73 Questions
Exam 19: Enzymes72 Questions
Exam 20: Introduction to Molecular Genetics84 Questions
Exam 21: Carbohydrate Metabolism75 Questions
Exam 22: Aerobic Respiration and Energy Production69 Questions
Exam 23: Fatty Acid Metabolism70 Questions
Select questions type
The drug "lithium" is often prescribed to treat mental illness.It is not the element lithium, but the ionic compound Li2CO3, that is actually administered.What is the name of this compound?
Free
(Multiple Choice)
5.0/5  (32)
(32)
Correct Answer:
D
In determining properties such as solubility, melting point, and boiling point, intramolecular forces are more important than intermolecular forces.
(True/False)
4.9/5  (30)
(30)
The ionic compound iron(II) sulfate is used in iron-containing supplements.What does the (II) in the name of this compound specifically indicate?
(Multiple Choice)
4.7/5  (35)
(35)
As a rule, a polar substance will be a good solvent for nonpolar solutes, and vice versa.
(True/False)
4.9/5  (43)
(43)
According to the VSEPR theory, what is the geometry (shape) around the phosphorus atom in the Lewis structure shown below? 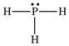
(Multiple Choice)
4.9/5  (42)
(42)
What is wrong with the Lewis structure shown for sulfur trioxide, SO3? 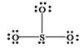
(Multiple Choice)
4.8/5  (44)
(44)
What kind of bonding exists in substances that consist of discrete molecules?
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following Lewis structures has a possible resonance structure? 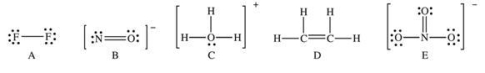
(Multiple Choice)
4.8/5  (39)
(39)
Showing 1 - 20 of 91
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)