Exam 3: Structure and Properties of Ionic and Covalent Compounds
Exam 1: Chemistry:methods and Measurement94 Questions
Exam 2: The Structure of the Atom and the Periodic Table109 Questions
Exam 3: Structure and Properties of Ionic and Covalent Compounds91 Questions
Exam 4: Calculations, Chemical Changes, and the Chemical Equation85 Questions
Exam 5: States of Matter- Gases, Liquids, and Solids75 Questions
Exam 6: Solutions67 Questions
Exam 7: Energy, Rate and Equilibrium61 Questions
Exam 8: Acids and Bases59 Questions
Exam 9: The Nucleus, Radioactivity, and Nuclear Medicine82 Questions
Exam 10: An Introduction to Organic Chemistry- the Saturated Hydrocarbons94 Questions
Exam 11: The Unsaturated Hydrocarbons- Alkenes, Alkynes, and Aromatics65 Questions
Exam 12: Alcohols, Phenols, Thiols, and Ethers65 Questions
Exam 13: Aldehydes and Ketones55 Questions
Exam 14: Carboxylic Acids and Carboxylic Acid Derivatives80 Questions
Exam 15: Amines and Amides80 Questions
Exam 16: Carbohydrates74 Questions
Exam 17: Lipids and Their Functions in Biochemical Systems80 Questions
Exam 18: Protein Structure and Function73 Questions
Exam 19: Enzymes72 Questions
Exam 20: Introduction to Molecular Genetics84 Questions
Exam 21: Carbohydrate Metabolism75 Questions
Exam 22: Aerobic Respiration and Energy Production69 Questions
Exam 23: Fatty Acid Metabolism70 Questions
Select questions type
The polyatomic ion known as the diphosphate ion is an important intermediate formed in metabolic processes.If the ionic compound calcium diphosphate has the formula Ca2P2O7, which of the following correctly represents the symbol for the diphosphate ion?
(Multiple Choice)
4.9/5  (33)
(33)
Emergency treatment of cardiac arrest victims sometimes involves injection of a calcium chloride (CaCl2) solution directly into the heart muscle.Which statement about the compound CaCl2 is FALSE?
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following is an essential feature of ionic bonding?
(Multiple Choice)
4.8/5  (36)
(36)
Formaldehyde is a covalent compound used as a preservative for biological specimens.Which statement concerning the Lewis structure of formaldehyde, shown below, is FALSE? 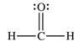
(Multiple Choice)
4.8/5  (38)
(38)
If the shape of a molecule is tetrahedral, what are the values of the bond angles?
(Multiple Choice)
4.9/5  (32)
(32)
Which combination of atoms is most likely to form an ionic compound if they are allowed to react with each other?
(Multiple Choice)
4.8/5  (37)
(37)
How many bonding electrons are present in the Lewis structure for the bicarbonate ion, shown below? 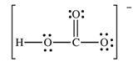
(Multiple Choice)
4.9/5  (38)
(38)
What does it mean if an atom is said to have a high electronegativity?
(Multiple Choice)
4.8/5  (30)
(30)
The hydrogen bonds between water and ammonia in a solution are intermolecular forces.
(True/False)
4.8/5  (36)
(36)
Six electrons shared between two atoms corresponds to a triple bond.
(True/False)
4.9/5  (31)
(31)
Showing 41 - 60 of 91
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)