Exam 3: Structure and Properties of Ionic and Covalent Compounds
Exam 1: Chemistry:methods and Measurement94 Questions
Exam 2: The Structure of the Atom and the Periodic Table109 Questions
Exam 3: Structure and Properties of Ionic and Covalent Compounds91 Questions
Exam 4: Calculations, Chemical Changes, and the Chemical Equation85 Questions
Exam 5: States of Matter- Gases, Liquids, and Solids75 Questions
Exam 6: Solutions67 Questions
Exam 7: Energy, Rate and Equilibrium61 Questions
Exam 8: Acids and Bases59 Questions
Exam 9: The Nucleus, Radioactivity, and Nuclear Medicine82 Questions
Exam 10: An Introduction to Organic Chemistry- the Saturated Hydrocarbons94 Questions
Exam 11: The Unsaturated Hydrocarbons- Alkenes, Alkynes, and Aromatics65 Questions
Exam 12: Alcohols, Phenols, Thiols, and Ethers65 Questions
Exam 13: Aldehydes and Ketones55 Questions
Exam 14: Carboxylic Acids and Carboxylic Acid Derivatives80 Questions
Exam 15: Amines and Amides80 Questions
Exam 16: Carbohydrates74 Questions
Exam 17: Lipids and Their Functions in Biochemical Systems80 Questions
Exam 18: Protein Structure and Function73 Questions
Exam 19: Enzymes72 Questions
Exam 20: Introduction to Molecular Genetics84 Questions
Exam 21: Carbohydrate Metabolism75 Questions
Exam 22: Aerobic Respiration and Energy Production69 Questions
Exam 23: Fatty Acid Metabolism70 Questions
Select questions type
Vehicle airbags inflate when the ionic compound sodium azide rapidly decomposes to the elements sodium and nitrogen.If the azide ion is a polyatomic ion with the formula N3−, what is the formula of sodium azide?
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following Lewis structures represent resonance forms of ozone, O3? 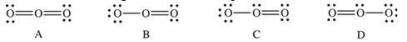
(Multiple Choice)
4.7/5  (34)
(34)
Draw the Lewis structures of F2, O2 and N2.Which statement is true?
(Multiple Choice)
4.8/5  (34)
(34)
In the molecule AX2, the central atom A has two lone pairs of electrons in addition to the two bond pairs in the A-X bonds.What is the shape of this molecule?
(Multiple Choice)
4.8/5  (35)
(35)
Because the C-H bond in methane is polar, the CH4 molecule will also be polar.
(True/False)
4.8/5  (35)
(35)
A molecule of deoxyribose, an essential part of DNA, contains five carbon atoms, ten hydrogen atoms, and four oxygen atoms.How would the formula of deoxyribose be represented?
(Multiple Choice)
4.9/5  (41)
(41)
Which compound contains a central atom with an exception to the octet rule known as an expanded octet?
(Multiple Choice)
4.8/5  (29)
(29)
Showing 81 - 91 of 91
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)