Exam 4: Covalent Compounds
Exam 1: Matter and Measurement87 Questions
Exam 2: Atoms and the Periodic Table95 Questions
Exam 3: Ionic Compounds100 Questions
Exam 4: Covalent Compounds101 Questions
Exam 5: Chemical Reactions98 Questions
Exam 6: Energy Changes,reaction Rates,and Equilibrium102 Questions
Exam 7: Gases,liquids,and Solids98 Questions
Exam 8: Solutions98 Questions
Exam 9: Acids and Bases108 Questions
Exam 10: Nuclear Chemistry93 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups123 Questions
Exam 12: Alkanes104 Questions
Exam 13: Unsaturated Hydrocarbons104 Questions
Exam 14: Organic Compounds That Contain Oxygen,halogen,or Sulfur112 Questions
Exam 15: The Three-Dimensional Shape of Molecules101 Questions
Exam 16: Aldehydes and Ketones114 Questions
Exam 17: Carboxylic Acids,esters,and Amides107 Questions
Exam 18: Amines and Neurotransmitters115 Questions
Exam 19: Lipids115 Questions
Exam 20: Carbohydrates100 Questions
Exam 21: Amino Acids,proteins,and Enzymes98 Questions
Exam 22: Nucleic Acids and Protein Synthesis98 Questions
Exam 23: Metabolism and Energy Production102 Questions
Exam 24: Carbohydrate,lipid,and Protein Metabolism99 Questions
Select questions type
Identify the diatomic element from the given choices.
Free
(Multiple Choice)
4.8/5  (39)
(39)
Correct Answer:
A
How many valence electrons are in a molecule of formaldehyde (CH2O)?
Free
(Multiple Choice)
4.9/5  (33)
(33)
Correct Answer:
B
Considering the electronegativity values indicated for each element,which covalent bond has the LEAST degree of polarity? 
Free
(Multiple Choice)
4.8/5  (41)
(41)
Correct Answer:
C
A double bond consists of four electrons shared between two atoms.
(True/False)
4.9/5  (34)
(34)
Predict the bond angles around the carbon atom in the structure of carbonic acid shown below. Don't forget to draw in lone pairs where needed to give octets. 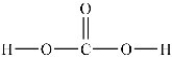
(Multiple Choice)
4.9/5  (40)
(40)
Rank the atoms Br,Cl,and F in order of increasing electronegativity.
(Multiple Choice)
5.0/5  (39)
(39)
How many lone pairs of electrons are present in the Lewis structure of ammonia,NH3?
(Multiple Choice)
4.9/5  (37)
(37)
The molecular shape around the boron atom in BCl3 is different than the molecular shape around the nitrogen atom in NCl3.
(True/False)
4.7/5  (40)
(40)
Which of the following compounds is classified as covalent?
(Multiple Choice)
4.8/5  (36)
(36)
Every atom must have an octet of electrons in order for a Lewis structure to be considered valid.
(True/False)
4.9/5  (30)
(30)
Which of the statements concerning chemical bonds is false?
(Multiple Choice)
4.9/5  (36)
(36)
A resonance hybrid is a composite of all resonance structures that spreads out electron pairs in multiple bonds and lone pairs.
(True/False)
4.9/5  (44)
(44)
How many nonbonded electron pairs are in the Lewis structure below? 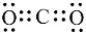
(Multiple Choice)
4.9/5  (41)
(41)
In Lewis structures,fluorine atoms generally do not obey the octet rule.
(True/False)
4.9/5  (36)
(36)
A molecule that contains only one polar bond is a polar molecule.
(True/False)
4.8/5  (33)
(33)
Showing 1 - 20 of 101
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)