Exam 1: Matter and Measurement
Exam 1: Matter and Measurement87 Questions
Exam 2: Atoms and the Periodic Table95 Questions
Exam 3: Ionic Compounds100 Questions
Exam 4: Covalent Compounds101 Questions
Exam 5: Chemical Reactions98 Questions
Exam 6: Energy Changes,reaction Rates,and Equilibrium102 Questions
Exam 7: Gases,liquids,and Solids98 Questions
Exam 8: Solutions98 Questions
Exam 9: Acids and Bases108 Questions
Exam 10: Nuclear Chemistry93 Questions
Exam 11: Introduction to Organic Molecules and Functional Groups123 Questions
Exam 12: Alkanes104 Questions
Exam 13: Unsaturated Hydrocarbons104 Questions
Exam 14: Organic Compounds That Contain Oxygen,halogen,or Sulfur112 Questions
Exam 15: The Three-Dimensional Shape of Molecules101 Questions
Exam 16: Aldehydes and Ketones114 Questions
Exam 17: Carboxylic Acids,esters,and Amides107 Questions
Exam 18: Amines and Neurotransmitters115 Questions
Exam 19: Lipids115 Questions
Exam 20: Carbohydrates100 Questions
Exam 21: Amino Acids,proteins,and Enzymes98 Questions
Exam 22: Nucleic Acids and Protein Synthesis98 Questions
Exam 23: Metabolism and Energy Production102 Questions
Exam 24: Carbohydrate,lipid,and Protein Metabolism99 Questions
Select questions type
A patient's urine sample has a density of 1.02 g/mL. If 1250 mL of urine was excreted by the patient in one day,what mass of urine was eliminated?
Free
(Multiple Choice)
4.8/5  (38)
(38)
Correct Answer:
A
Carry out the following calculation and report the answer using the proper number of significant figures: 38.251 × 73.1
Free
(Multiple Choice)
4.9/5  (31)
(31)
Correct Answer:
E
When 0.022189 is correctly rounded to two significant figures the number becomes ________.
Free
(Multiple Choice)
4.9/5  (28)
(28)
Correct Answer:
B
PVC plastic,which is used in pipes,is an example of a synthetic material.
(True/False)
4.8/5  (45)
(45)
When a piece of magnesium (density = 1.738 g/mL)is placed in a container of liquid carbon tetrachloride (density = 1.59 g/mL),the piece of magnesium will float on top of the carbon tetrachloride.
(True/False)
4.7/5  (27)
(27)
A zero counts as a significant figure when it occurs at the end of a number that contains a decimal point.
(True/False)
4.9/5  (36)
(36)
The measurement 10.3 cm has more significant figures than the measurement 10.3 m.
(True/False)
4.7/5  (38)
(38)
The two conversion factors for the equality 1 in = 2.54 cm are properly shown below. 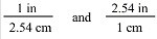
(True/False)
4.8/5  (34)
(34)
An oven is set for a temperature of 298 °F. What is the oven temperature in K?
(Multiple Choice)
4.7/5  (32)
(32)
When the liquid carbon tetrachloride (density = 1.59 g/mL)is added to water,the top layer will be the water layer.
(True/False)
4.7/5  (31)
(31)
If a package of nuts weighs 41.3 oz,what is the mass of the package expressed in milligrams?
(Multiple Choice)
4.7/5  (30)
(30)
If a 185-lb patient is prescribed 145 mg of the cholesterol lowering drug Tricor daily,what dosage is the patient receiving in mg/kg of his body weight?
(Multiple Choice)
4.9/5  (38)
(38)
A mixture can be separated into its components by physical changes.
(True/False)
4.9/5  (34)
(34)
The water molecules in this image are best described as being in the liquid state. 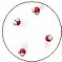
(True/False)
4.9/5  (37)
(37)
Which volume has the most uncertainty associated with the measurement?
(Multiple Choice)
4.8/5  (34)
(34)
What is the mass in kilograms of an individual who weighs 197 lb?
(Multiple Choice)
4.9/5  (27)
(27)
Showing 1 - 20 of 87
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)