Exam 14: Oxidation-Reduction Reactions
Exam 1: Foundations30 Questions
Exam 2: Measurement25 Questions
Exam 3: Atoms30 Questions
Exam 4: Light and Electronic Structure30 Questions
Exam 5: Chemical Bonds Compounds56 Questions
Exam 6: Chemical Reactions47 Questions
Exam 7: Mass Stoichiometry44 Questions
Exam 8: Energy39 Questions
Exam 9: Covalent Bonding and Molecules30 Questions
Exam 10: Solids, Liquids, and Gases30 Questions
Exam 11: Solutions30 Questions
Exam 12: Acids Bases30 Questions
Exam 13: Reaction Rates Equilibrium40 Questions
Exam 14: Oxidation-Reduction Reactions52 Questions
Exam 15: Organic Chemistry and Biomolecules65 Questions
Exam 16: Nuclear Chemistry52 Questions
Select questions type
Which pair of half-reactions could be used to electroplate silver onto iron?
(Multiple Choice)
4.9/5  (41)
(41)
If the accompanying drawing of an electrochemical cell represents an iron-nickel cell, use the partial activity series included to select the half-reaction represented by the LEFT side of the cell. 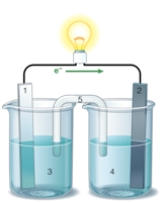 Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
(Multiple Choice)
4.8/5  (38)
(38)
In the context of chemical reactions, oxidation refers to the:
(Multiple Choice)
4.8/5  (34)
(34)
What are the coefficients of the balanced equation that represents the reaction in a magnesium/iron electrochemical cell?
Mg (s) + Fe2+ (aq) Fe (s) + Mg2+ (aq)
(Multiple Choice)
4.8/5  (31)
(31)
Using the accompanying drawing of an electrochemical cell, identify the components labeled 1, 2, and 5, respectively. 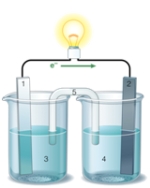
(Multiple Choice)
4.9/5  (46)
(46)
Use the partial activity series included to determine which reaction will NOT occur spontaneously. Zn Zn2+ + 2e-
Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
(Multiple Choice)
4.9/5  (35)
(35)
Consider an electrochemical cell consisting of zinc and cobalt half-cells. Use the partial activity series included to determine the direction in which the electrons in the wire will flow.
Zn Zn2+ + 2e-
Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
(Multiple Choice)
4.9/5  (33)
(33)
Knowing that sodium and potassium react violently with water, predict which element will also react with water.
(Multiple Choice)
4.8/5  (41)
(41)
What is the oxidation number of sulfur in the sulfite ion, SO32-?
(Multiple Choice)
4.7/5  (38)
(38)
Given the accompanying partial activity series, which element(s) can reduce copper(II) to elemental copper?
Zn Zn2+ + 2e-
Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
(Multiple Choice)
4.9/5  (39)
(39)
The oxidation half-reaction for the reaction of zinc with hydrochloric acid is:
(Multiple Choice)
4.7/5  (29)
(29)
What is the oxidation number of carbon in carbon monoxide, CO?
(Multiple Choice)
4.8/5  (38)
(38)
If the accompanying drawing of an electrochemical cell represents an iron-nickel cell, use the partial activity series included to identify the components labeled 1, 2, 3, and 4 respectively. 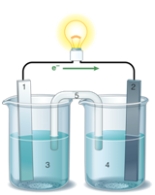 Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
Zn Zn2+ + 2e- Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
(Multiple Choice)
4.8/5  (28)
(28)
What are the coefficients of the balanced equation that represents the reaction in a magnesium/aluminum electrochemical cell?
Mg (s) + Al3+ (aq) Al (s) + Mg2+ (aq)
(Multiple Choice)
4.7/5  (43)
(43)
What is the oxidation number of sulfur in sulfur trioxide, SO3?
(Multiple Choice)
4.8/5  (29)
(29)
Write the balanced net ionic equation for the reaction consisting of the two half-reactions given.
Cu Cu2+ + 2e-
Au3+ + 3e- Au
(Multiple Choice)
4.8/5  (35)
(35)
Given the accompanying partial activity series, predict which element or ion will react with cobalt, Co.
Zn Zn2+ + 2e-
Fe Fe2+ + 2e-
Co Co2+ + 2e-
Ni Ni2+ + 2e-
Cu Cu2+ + 2e-
(Multiple Choice)
4.8/5  (50)
(50)
Given the accompanying partial activity series, which element(s) will NOT react with acid?
Zn Zn2+ + 2e-
Fe Fe2+ + 2e-
Ni Ni2+ + 2e-
H2 2H+ + 2e-
Cu Cu2+ + 2e-
Ag Ag+ + e-
(Multiple Choice)
4.8/5  (48)
(48)
Showing 21 - 40 of 52
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)