Exam 1: Covalent Bonding and Shapes of Molecules
Exam 1: Covalent Bonding and Shapes of Molecules95 Questions
Exam 2: Alkanes and Cycloalkanes77 Questions
Exam 3: Stereoisomerism and Chirality75 Questions
Exam 4: Acids and Bases75 Questions
Exam 5: Alkenes: Bonding, Nomenclature, and Properties70 Questions
Exam 6: Reactions of Alkenes79 Questions
Exam 7: Alkynes80 Questions
Exam 8: Haloalkanes, Halogenation, and Radical Reactions58 Questions
Exam 9: Nucleophilic Substitution and Beta-Elimination89 Questions
Exam 10: Alcohols78 Questions
Exam 11: Ethers, Epoxides, and Sulfides71 Questions
Exam 12: Infrared Spectroscopy44 Questions
Exam 13: Nuclear Magnetic Resonance Spectroscopy73 Questions
Exam 14: Mass Spectrometry39 Questions
Exam 15: An Introduction to Organometallic Compounds45 Questions
Exam 16: Aldehydes and Ketones94 Questions
Exam 17: Carboxylic Acids51 Questions
Exam 18: Functional Derivatives of Carboxylic Acids88 Questions
Exam 19: Enolate Anions and Enamines70 Questions
Exam 20: Dienes, Conjugated Systems, and Pericyclic Reactions44 Questions
Exam 21: Benzene and the Concept of Aromaticity59 Questions
Exam 22: Reactions of Benzene and Its Derivatives83 Questions
Select questions type
Which of the following elements has the highest electronegativity?
(Multiple Choice)
4.7/5  (35)
(35)
Which atomic orbitals overlap to form the C=O bond of acetone, (CH3)2C=O?
(Multiple Choice)
4.8/5  (31)
(31)
Which of the following best represents an sp3 hybridized atomic orbital containing the lone pair of electrons of ammonia, NH3? 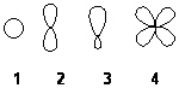
(Multiple Choice)
4.7/5  (35)
(35)
What is the approximate value of the H-C-H bond angles in a methyl cation, CH3+?
(Multiple Choice)
4.9/5  (28)
(28)
Which atomic orbitals overlap to form the carbon-hydrogen s bonding molecular orbitals of ethyne, HCºCH?
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following statements is not true regarding resonance structures?
(Multiple Choice)
4.8/5  (32)
(32)
What is the ground-state electronic configuration of a sodium cation (sodium: atomic number 11)?
(Multiple Choice)
4.9/5  (45)
(45)
Which of the following best represents the shape of the 2s atomic orbital of carbon? 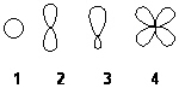
(Multiple Choice)
4.8/5  (36)
(36)
Showing 81 - 95 of 95
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)