Exam 22: The Elements in Nature and Industry
Exam 1: Keys to the Study of Chemistry68 Questions
Exam 2: The Components of Matter104 Questions
Exam 3: Stoichiometry of Formulas and Equations96 Questions
Exam 4: Three Major Classes of Chemical Reactions105 Questions
Exam 5: Gases and the Kinetic-Molecular Theory103 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change79 Questions
Exam 7: Quantum Theory and Atomic Structure74 Questions
Exam 8: Electron Configuration and Chemical Periodicity81 Questions
Exam 9: Models of Chemical Bonding73 Questions
Exam 10: The Shapes of Molecules108 Questions
Exam 11: Theories of Covalent Bonding56 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes97 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids98 Questions
Exam 14: Periodic Patterns in the Main-Group Elements111 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon113 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions89 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions102 Questions
Exam 18: Acid-Base Equilibria106 Questions
Exam 19: Ionic Equilibria in Aqueous Systems115 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry56 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications90 Questions
Select questions type
Hydrometallurgy uses __________________ to separate a metal from its ore.
(Multiple Choice)
4.8/5  (42)
(42)
What gas is produced at the anode in the Downs cell, in which molten NaCl is electrolyzed?
(Short Answer)
4.8/5  (34)
(34)
Aluminum and magnesium form are light metals which are used in many structural applications. Although they are highly active metals (as their electrode potentials suggest), they can often be used without the use of paint or other applied finishes to protect them against corrosion. How is this possible?
(Essay)
4.7/5  (45)
(45)
Which one of the following species or compounds reacts at the cathode in the Hall-Héroult process?
(Multiple Choice)
4.8/5  (28)
(28)
The atmosphere contains about 700. billion metric tons of carbon in the form of carbon dioxide. In the carbon cycle, about 200. billion metric tons of carbon (as carbon dioxide) enters the atmosphere each year. Assuming that 200. billion metric tons of carbon also leaves the atmosphere annually, how many years does the average carbon atom spend in the atmosphere in each cycle?
(Multiple Choice)
4.9/5  (38)
(38)
Calcium oxide is added to molten iron in the production of carbon steel in order to
(Multiple Choice)
4.8/5  (44)
(44)
In the industrial electrolysis of aqueous NaCl (the chlor-alkali process), the modern trend is toward the use of cells incorporating polymer membranes to separate the anode and cathode solutions.
(True/False)
4.7/5  (36)
(36)
Pyrometallurgy uses __________________ to separate a metal from its ore.
(Multiple Choice)
4.8/5  (32)
(32)
The ______________ process uses a boiling 30% sodium hydroxide solution to treat bauxite.
(Multiple Choice)
4.9/5  (45)
(45)
Sulfur trioxide is the anhydride of sulfuric acid. However, SO3 is not added directly to water during the synthesis of sulfuric acid because
(Multiple Choice)
4.8/5  (38)
(38)
The leaching of gold can be represented by the equation 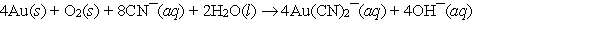 In this reaction, identify the oxidant, the reductant and the oxidation numbers of the elements which change.
In this reaction, identify the oxidant, the reductant and the oxidation numbers of the elements which change.
(Essay)
4.8/5  (42)
(42)
What gas is produced during the Hall-Héroult process for production of aluminum?
(Multiple Choice)
4.8/5  (32)
(32)
Electrolysis is used as the last step in isolating pure __________________.
(Multiple Choice)
4.8/5  (41)
(41)
The most common source for commercial production of sodium is called
(Multiple Choice)
4.9/5  (37)
(37)
Showing 41 - 56 of 56
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)