Exam 22: The Elements in Nature and Industry
Exam 1: Keys to the Study of Chemistry68 Questions
Exam 2: The Components of Matter104 Questions
Exam 3: Stoichiometry of Formulas and Equations96 Questions
Exam 4: Three Major Classes of Chemical Reactions105 Questions
Exam 5: Gases and the Kinetic-Molecular Theory103 Questions
Exam 6: Thermochemistry: Energy Flow and Chemical Change79 Questions
Exam 7: Quantum Theory and Atomic Structure74 Questions
Exam 8: Electron Configuration and Chemical Periodicity81 Questions
Exam 9: Models of Chemical Bonding73 Questions
Exam 10: The Shapes of Molecules108 Questions
Exam 11: Theories of Covalent Bonding56 Questions
Exam 12: Intermolecular Forces: Liquids, Solids, and Phase Changes97 Questions
Exam 13: The Properties of Mixtures: Solutions and Colloids98 Questions
Exam 14: Periodic Patterns in the Main-Group Elements111 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon113 Questions
Exam 16: Kinetics: Rates and Mechanisms of Chemical Reactions89 Questions
Exam 17: Equilibrium: the Extent of Chemical Reactions102 Questions
Exam 18: Acid-Base Equilibria106 Questions
Exam 19: Ionic Equilibria in Aqueous Systems115 Questions
Exam 20: Thermodynamics: Entropy, Free Energy, and the Direction of Chemical Reactions85 Questions
Exam 21: Electrochemistry: Chemical Change and Electrical Work102 Questions
Exam 22: The Elements in Nature and Industry56 Questions
Exam 23: The Transition Elements and Their Coordination Compounds92 Questions
Exam 24: Nuclear Reactions and Their Applications90 Questions
Select questions type
The main effect of the biosphere on the chemistry of the earth's crust has been to
Free
(Multiple Choice)
4.9/5  (37)
(37)
Correct Answer:
B
The purpose of anodizing aluminum is to remove the oxide layer from the metal's surface.
Free
(True/False)
4.9/5  (45)
(45)
Correct Answer:
False
The final step of the purification of copper involves electrorefining in which copper is separated from nickel and iron by being reduced at the cathode of a cell. Why are nickel and iron not reduced?
Free
(Multiple Choice)
5.0/5  (31)
(31)
Correct Answer:
B
The process that selectively extracts a metal from its ore, by dissolving it, is called
(Multiple Choice)
4.8/5  (45)
(45)
The process in which a gaseous substance is converted into a condensed, more usable chemical substance is called
(Multiple Choice)
4.8/5  (38)
(38)
The process used to produce silicon with a purity of more than 99.999999% is called
(Multiple Choice)
4.8/5  (38)
(38)
The alkali metals are isolated from non-aqueous systems. Why is this necessary?
(Multiple Choice)
4.9/5  (31)
(31)
Transition elements from the left side of the periodic table are generally found as
(Multiple Choice)
4.7/5  (36)
(36)
Lightning, fires and other atmospheric processes result in the formation of nitric acid, HNO3, from atmospheric N2, O2 and H2O. Write balanced chemical equations to represent the three reactions involved in the formation of HNO3 by this route.
(Essay)
4.8/5  (42)
(42)
Nitrogen fixation occurs through atmospheric, industrial, and biological processes. Which of these fixes the most nitrogen?
(Multiple Choice)
4.8/5  (38)
(38)
Potassium is produced industrially by the reaction Na(l) + K+(l) 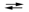 Na+(l) + K(g). The reaction is carried out at 850 C, but the equilibrium Kc constant strongly favors the reactants. Explain how the conditions are manipulated to ensure a good yield of potassium despite the small value of Kc.
Na+(l) + K(g). The reaction is carried out at 850 C, but the equilibrium Kc constant strongly favors the reactants. Explain how the conditions are manipulated to ensure a good yield of potassium despite the small value of Kc.
(Essay)
4.7/5  (30)
(30)
The most common source for commercial production of aluminum is called
(Multiple Choice)
4.9/5  (47)
(47)
The bond energy in the hydrogen molecule (H2) is greater than that of the tritium molecule (T2).
(True/False)
4.8/5  (40)
(40)
Compared to the asbestos diaphragm-cell used in the chlor-alkali process, list
A) one main advantage and one disadvantage of mercury cells.
B) two important advantages of polymer membrane-cells.
(Essay)
4.8/5  (36)
(36)
Which of the following reactions is a good small-scale laboratory method for preparation of hydrogen?
(Multiple Choice)
4.9/5  (34)
(34)
Showing 1 - 20 of 56
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)